The Government of India has invited public input on new draft regulations for Clinical Electrical Thermometers, with the deadline for feedback set as December 30, 2024. Initiated by the Department of Consumer Affairs, these proposals align with recommendations from the International Organization of Legal Metrology (OIML) and were made available for public consultation on November 29, 2024.
Objective of the Proposed Regulations
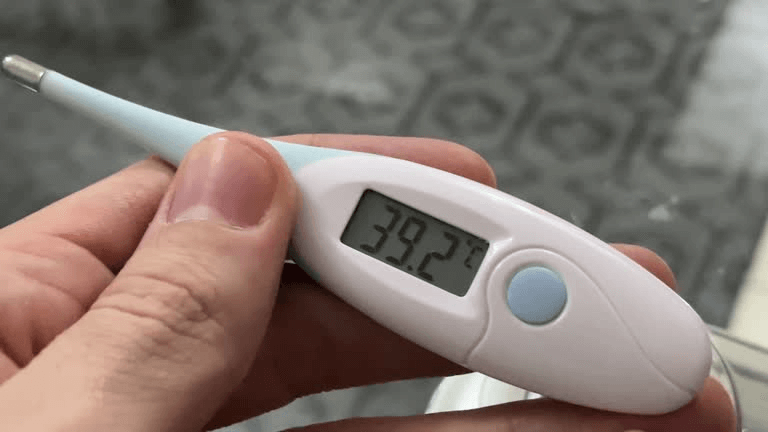
The primary goal of these rules is to ensure accuracy and reliability in Clinical Electrical Thermometers. As essential tools in health diagnostics, these devices must deliver precise readings to enable proper treatment. By implementing these standards, the government aims to safeguard the health of both humans and animals.
Key Features of the Proposed Regulations
- Mandatory Verification and Stamping:
All thermometers will be required to undergo verification and bear a stamp of compliance. - Accuracy Standards:
Devices must meet stringent accuracy requirements to ensure their reliability in diverse settings. - Applicability:
The regulations will cover thermometers used in homes, hospitals, and industries, ensuring consistency across all environments.
Enhancing Consumer Trust
The adoption of these regulations is expected to significantly boost consumer confidence. With reliable thermometer measurements, individuals will feel more assured about health assessments. This is especially critical for detecting conditions like fever and hypothermia, where accuracy can make a vital difference.
Role of the Legal Metrology Department
The Legal Metrology Department, responsible for ensuring the accuracy of measuring instruments in India, will oversee the implementation of these rules. The amendments will modernize existing regulations, aligning them with global standards and reinforcing consumer rights.
Accurate thermometer readings are indispensable for effective diagnosis and treatment. By standardizing the performance of Clinical Electrical Thermometers, the proposed regulations will not only enhance healthcare outcomes but also contribute to a more trustworthy and equitable health system.